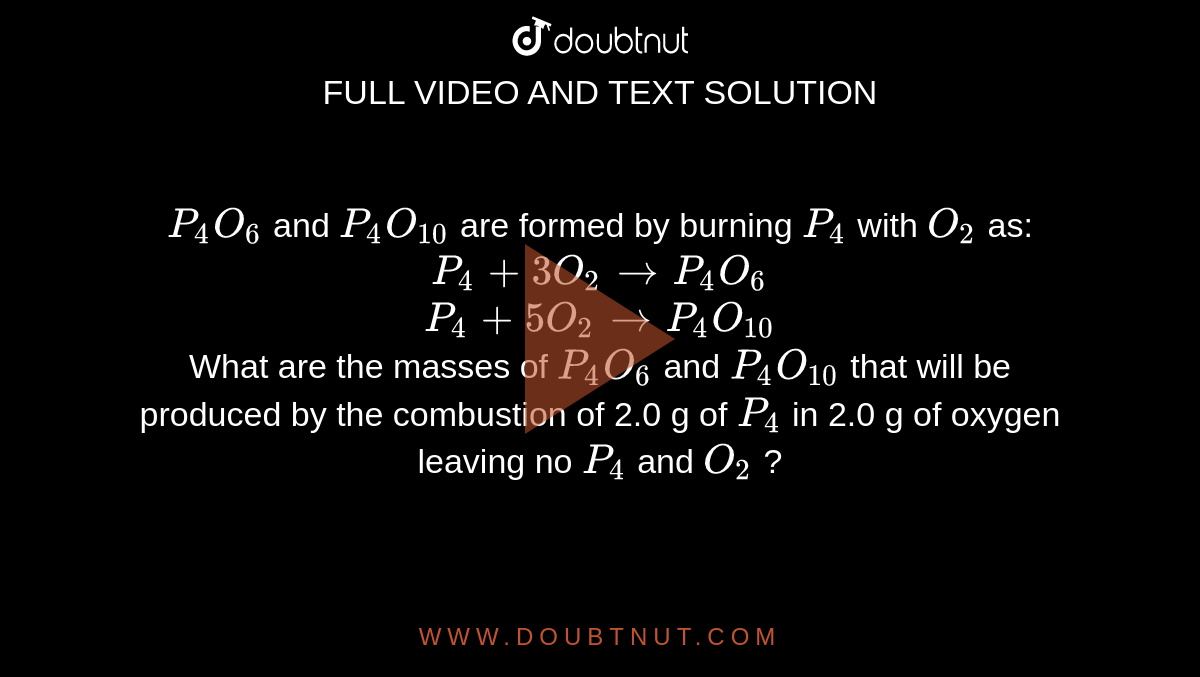
For the given reaction P4 +5o2 ---- p4O10 if 31 g of P4 is reacted with excess of oxygen the - Chemistry - - 14702803 | Meritnation.com
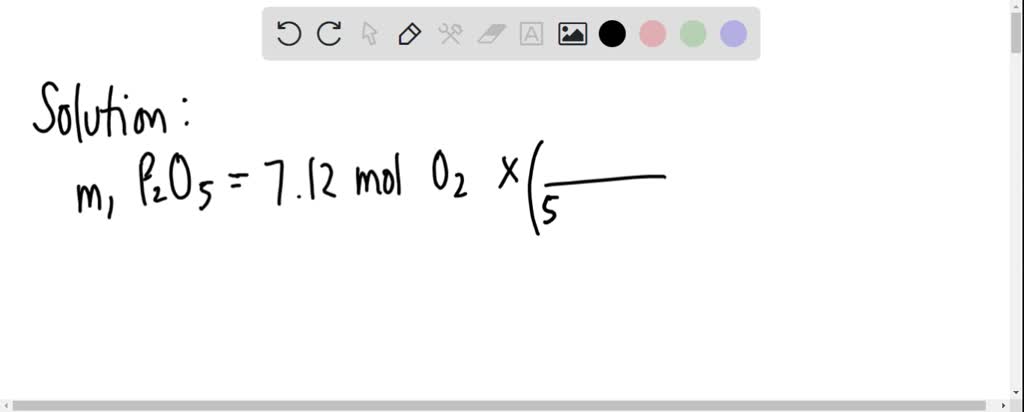
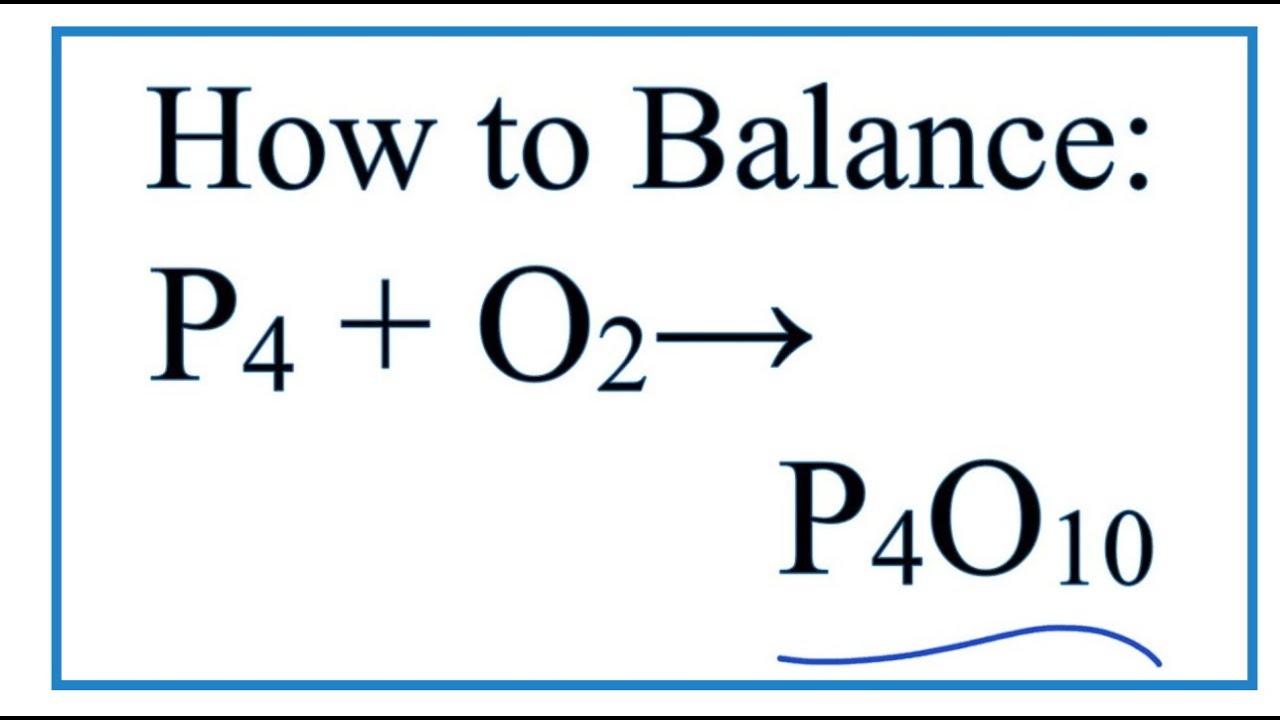
SOLVED: According to this equation, how much P4O10 can be produced from 8.32 g of phosphorus? 4 P + 5 O2 → P4O10
11 The mass of P4O10 produce if 440 gm of P4S is mixed with 384 gm of O2 Is given, P4S3 + O2 — > P4O10 + SO2 OPTIONS >> 568gm, 426gm , 284gm, 396gm.
a) What is the limiting reactant when 0.200 mol of P4 and 0.200mol of O2 react according to: P4 + 5O2 = P4O10 (b) Calculate the percent yield if 10.0 g of